Financial Analysis
July 12, 2022
Crypto
July 12, 2022Navigating the intricate terrain of healthcare quality, this study unveils the tapestry of challenges confronting the medical laboratory as it strives for ISO 15189 accreditation within the distinctive setting of Gozo General Hospital.
In the pursuit of understanding the pivotal role of ISO 15189 within Gozo General Hospital, this chapter delves into a comprehensive review of related literature. The focus extends beyond merely outlining the functions of medical laboratories; it scrutinizes the significance of these functions in the broader context of the healthcare system. Additionally, the chapter sheds light on factors that exert influence on the competence and quality of medical laboratories, while also addressing their roles, implementation processes, and the challenges they encounter.
Learn More About Medical Science
Towards the culmination of this chapter, a dedicated section presents an effective strategy to navigate the challenges posed by ISO 15189. This strategy is underpinned by a robust theoretical framework, providing a practical approach to enhancing the hospital's compliance with the standard. To further enhance the reader's understanding, a conceptual framework is introduced just before the chapter concludes, synthesizing the identified factors.
Explore More About Challenges in Medical Science
Section 2.1 serves as a preview, offering the reader insight into the upcoming topics and, crucially, explaining the rationale behind their inclusion. This preview not only outlines the planned coverage but also articulates how each topic contributes to the overarching objectives of the study, facilitating a clear understanding of the journey ahead.
Functions Performed In Medical Laboratories
Medical laboratories serve as indispensable facilities for conducting a myriad of medical tests essential for comprehending the health conditions of patients and facilitating treatment, diagnosis, and disease prevention (Neubauer and Heurix, 2011). In medical research and clinical trials, the significance of a clinical or medical laboratory cannot be overstated.
The multifaceted functions of medical laboratories encompass the examination and analysis of blood, tissue, urine, body fluids, blood grouping, matching, screening, and RH typing (Gargis et al., 2012). Furthermore, these laboratories perform qualitative and quantitative testing crucial for diverse research purposes (Harmening, 2018). Qualitative analyses unveil the constituents and mixtures of substances. In contrast, quantitative analyses delve into the precise measurement and analysis of amounts, ratios, and proportions for testing and experimentation (Bishop, Fody, and Schoeff, 2013). The pivotal role of medical laboratories in conducting medical tests and research is underscored in various studies, positioning them as the bedrock of medical research and studies (Shieh, Wu, and Huang, 2010).
Medical laboratories conduct various investigations and studies across different areas of laboratory science, including haematology, clinical chemistry, coagulation, transfusion medicine, immunology, histology, microbiology, and molecular diagnostics (McGinnis, 2012). These areas collectively contribute to developing life-saving and curative medicines, emphasizing the role of medical laboratories as the cornerstone for experiments, testing, and research in drug and medicine development. Consequently, it becomes imperative for medical laboratories to regularly update their methodologies and equipment to ensure effective functioning (Shieh, Wu, and Huang, 2010).
In summary, medical laboratories play a pivotal role by performing five core functions: conducting research related to public health, responding to communicable diseases during emergencies, performing diagnostic testing, managing integrated disease data, and ensuring environmental health and food safety (Amato et al., 2013).
Significance Of Quality And Competence In The Healthcare Sector
Quality and competence within the healthcare sector are emblematic of applying, implementing, and demonstrating a tailored set of skills, knowledge, and behaviours in clinical environments (Morgan, Ensor, and Waters, 2016). These attributes are focused on precise behaviours and actions rooted in extensive knowledge and high skills, ensuring the delivery of standardized healthcare services in both routine and critical scenarios (Büyüközkan, Çifçi, and Güleryüz, 2011). Understanding the significance of quality and competence in healthcare is pivotal as it directly relates to service quality and patient care across various dimensions. With the healthcare sector rapidly evolving due to technological advancements and increased demands for quality, organizations recognise the importance of these attributes in providing effective treatments and healthcare services (Berendes et al., 2011).
Healthcare organizations acknowledge the paramount need for competence, particularly as the industry focuses on delivering patient-centric value through proficient staff members, including nurses, doctors, and healthcare facilitators. This shift emphasizes the importance of technology, effective treatments, and accurate reporting in hospitals (Morgan, Ensor, and Waters, 2016). Competency and quality play a crucial role in addressing challenges within the clinical environment, enabling staff members to implement effective strategies that maintain high-quality healthcare services and treat patients with expertise (Chahal and Bala, 2012). The changing nature of healthcare delivery prompts organizations to review and develop competency programs, assessment objectives, and strategies to cultivate and sustain an advanced and skilled workforce, ensuring the ongoing effectiveness of treatments and healthcare services (Büyüközkan, Çifçi, and Güleryüz, 2011).
The significance of quality and competence is further underscored by the various laws and regulations imposed in the healthcare sector to ensure the delivery of high-quality medical treatments and healthcare services (Das, 2011). Additionally, a set of core competencies mandated by regulations guide healthcare organizations. These competencies encompass providing patient-centred care, collaborating in interdisciplinary teams with an integrated approach, applying evidence and experience-based practices, developing consistent strategies for advancement and treatment quality improvement, and utilizing informatics for effective communication, risk mitigation, error prevention, and decision-making using information technology (Alizadeh and Chavan, 2016). These core competencies exemplify the paramount importance of quality and competence across the entire healthcare sector and are actively adhered to by various healthcare organizations.
Impact of Quality and Competence in the Medical Laboratories
Quality and competence within medical laboratories extend beyond individual practices, encompassing the equipment used, clinical settings, and testing and research methodologies (Mosadeghrad, 2012). In the contemporary era, medical laboratories prioritize laboratory automation, molecular diagnostics, laboratory consolidation, imaging analysis, and clinical laboratory accreditation. These priorities aim to enhance the quality and competence of patient care while conducting diverse tests and research on clinical specimens, including urine and blood tests (Talib, Rahman, and Azam, 2011).
Key indicators influencing quality in medical laboratories are intertwined with broader healthcare goals, including population health, equal opportunities for healthcare access, effective delivery of healthcare services, and the accessibility of systems. Additionally, healthcare results and reporting mechanisms contribute significantly to the overall quality impact (Plebani, 2012). Factors such as population lifestyle, genetics, and environmental considerations substantially influence population health, emphasizing the interconnectedness of various elements in the healthcare landscape (Plebani et al., 2013). Furthermore, regulations governing quality and competence in medical laboratories, anchored in accreditation, quality monitoring, certification, patients' rights, and standard operational processes, also play a pivotal role in shaping quality standards within the healthcare sector (Gargis et al., 2012).
Laboratory medicine is hailed as the backbone of the healthcare sector, profoundly impacting medical treatments, diagnoses, and prevention strategies. Its influence permeates the decision-making processes within healthcare organizations, determining operational costs and treatment approaches for patients (Beastall et al., 2005).
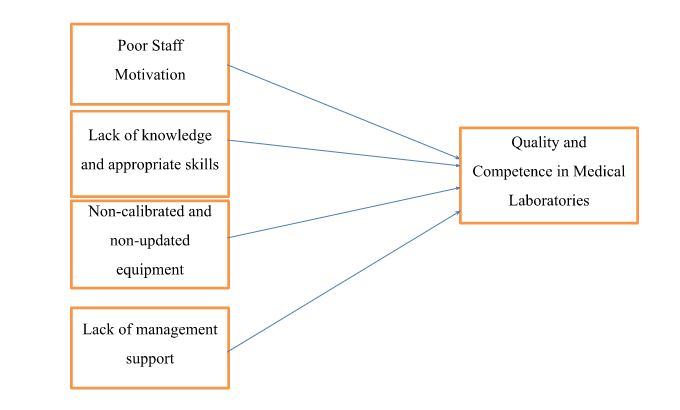
The quality and competence of medical laboratory services are subject to various influencing factors (Mosadeghrad, 2014). Despite stringent regulations and laws emphasizing consistent adherence to quality and competence in the healthcare sector, certain influential factors can compromise these standards. Notably, poor staff motivation, insufficient knowledge and skills, the utilization of non-calibrated and outdated equipment in medical laboratories, and a lack of management support emerge as critical elements influencing the overall quality and competence in these vital healthcare facilities (Mosadeghrad, 2014).
Poor Staff Motivation
Staff motivation levels play a pivotal role in shaping the quality and competence within medical laboratories, directly influencing the workforce engaged in the healthcare sector, particularly in clinical laboratories (Brown and Busman, 2003). Numerous studies have underscored the negative correlation between poor staff motivation and the efficiency of working with quality, leading to a potential decrease in laboratory competence levels (Chhea, Warren, and Manderson, 2010). This decline in efficiency and competence has far-reaching implications for the service quality during research and testing, ultimately affecting the overall functioning of medical laboratories, where the effectiveness of their functions is inherently tied to the motivation and commitment of the staff.
Lack of Knowledge and Appropriate Skills
Possessing appropriate skills and in-depth knowledge of medical functions is paramount for the seamless operation of medical laboratories (Houghton et al., 2012). Conversely, a lack of knowledge and relevant skills significantly impacts the quality and competence levels within these laboratories, posing a risk to the delivery of medical services and the overall competence of the workforce (Rosenzweig et al., 2012). This deficiency can result in inefficiencies in conducting research and tests, posing serious implications for healthcare organizations and the patients receiving treatment within these institutions (Houghton et al., 2012).
Using Non-Calibrated and Non-Updated Equipment
Non-calibrated and outdated equipment in medical laboratories stands out as a factor detrimental to quality and competence in a clinical setting. Equipment that is not aligned with the clinical environment and is aged significantly cannot deliver accurate outcomes. This mismatch can decrease efficiency and effectiveness in performing functions within medical laboratories, ultimately compromising the overall quality and generating inappropriate results in the form of numerous errors during tests and research activities in a clinical setting (Ferrari et al., 2010).
Lack of Management Support
Management support holds significant sway in the healthcare sector, fostering effective functions within medical laboratories and enhancing staff efficiency (Olmsted et al., 2010). Conversely, a lack of management support can manifest as inadequate budgets, insufficient provision of necessary equipment and tools, and neglect of staff satisfaction in clinical laboratories. These shortcomings directly impinge on the quality and competence within medical laboratories, disrupting the accuracy of laboratory functions (Marinucci et al., 2013). Furthermore, the absence of management support can lead to ineffective treatments, underscoring management's critical role in shaping the healthcare landscape (Olmsted et al., 2010).
Role of International Standards in the Laboratory Operations
According to a study conducted by Antonelli et al. (2017), operations in medical laboratories necessitate careful handling of samples, particularly considering that these samples often originate from patients afflicted with various diseases. The study emphasizes the importance of ensuring that laboratory staff is shielded from exposure to samples carrying serious diseases such as Hepatitis and AIDS. Another study by Chapman and Himmerick (2017) highlights reported cases where medical lab technicians were exposed to samples of severe diseases, underscoring the need for a management environment that prioritizes the safety and well-being of laboratory staff.
In alignment with these findings, Sikaris (2017) emphasizes the pivotal role of medical laboratories in testing patient samples, where the resultant reports significantly influence healthcare professionals' diagnosis and treatment decisions. Maintaining the quality and competence of medical laboratories becomes paramount in such a context, as the patient's medication process relies heavily on the accuracy of laboratory reports. Plebani et al. (2015) also highlight the importance of adhering to standards established by the International Standardization Organisation (ISO), emphasizing the need for organizations to follow defined ISO standards in their respective divisions.
The focus on ISO 15189, as identified in the study, centres on preserving the quality and competencies of medical laboratories. The study outlines a four-step process that aligns with ISO requirements. Commencing with strategic management, the process moves through design, planning, and control stages. An analytical phase assesses whether the organizational strategy contributes to the quality management system. If gaps for improvement are identified, the organization's management should consider and redesign their strategy accordingly. The last stage involves continuous strategic planning, ensuring ongoing functionality, process evaluation, and improvement in management practices. Critically examining and questioning the assumptions behind cited studies could provide a nuanced understanding of the subject matter.
Implementation of ISO 15189 in Medical Laboratories
According to the study that has been conducted by (Antonelli et al., 2017), it has been observed that there are some requirements that the medical laboratory should fulfil before the implementation of ISO 15189. These requirements are majorly the responsibility of the managerial personnel of the organization as it has been observed that the major requirement of ISO15189 is the organization and management that in a case of misconduct, there should be some of the personnel who have been already identified as the responsibility for any misconduct. The organisation's policies, rules, and regulations should be designed under the supervision of the person who will take all the responsibilities later (Misganaw, 2016). The second requirement for implementing ISO 15189 is the quality management system, where the documentation process should be managed suitably, and the organisation's management should predefine the roles of each person in the lab. There should be document control and review of a contract, as the documents in any lab regarding their legal issues or the patients should be handled with care and organisation. Moreover, the lab's contract should also be reviewed periodically to check for any need for amendment.
Furthermore, it has also been observed that ISO 15189 has one of the requirements regarding laboratory referrals. For example, a lab has been referring the patient to another lab. It should be considered that both the referring and the referral lab should meet the criteria of ISO 15189. In another study conducted by (Oosterhuis et al., 2018), it was observed that there should be a predefined criterion in the lab for the acceptance, rejection, and evaluation of the material they have been procuring from outside resources. Staff should also be available to provide advisory services for the patients on what service of the laboratory they should be using, what the procedure is, and how it would benefit them. Another requirement of ISO 15189 is that there should be a significant mechanism available in the lab to rectify the problems that patients have been facing in the laboratory (Nasser et al., 2018). The lab should maintain a record of the complaints, which should contain the details regarding the root cause, its analysis, and the corrective action the management has taken against the complaint. There should be preventive action in addition to corrective action; the management of the lab should have their strategy amended so that a similar issue does not occur again. Furthermore, the management should review the lab's process and operation and conduct an internal audit. There are lots of “shoulds” here, but I suppose the subject matter justifies them.
In addition to the managerial requirement, there are some technical requirements as well, as it has been observed that the lab obtaining the ISO 15189 should have technically trained professionals to deal with the equipment at the lab. The lab's operations should be executed in a way that should not cause any harm to the environment. The laboratory's equipment should be updated, properly maintained, and periodically checked for its functioning. Any sample coming to the medical lab has three stages: pre-examination, examination, and post-examination; all three stages should be handled with care as every patient should be provided the right service correctly, and their report should be error-free. Reporting the result is one of the most important stages in the whole process of medical labs as no report should be missed or contain errors in case the patient would have to face serious consequences. Very good.
Challenges of Accreditation of ISO 15189 Faced by Medical Laboratories
According to the study that was conducted using the wrong form of Harvard citation (Jones and Jackson, 2016), it has been observed that there are some challenges that the medical laboratories have been facing while obtaining accreditation of ISO 15189 from the international standardization organization. As there are two types of requirements for the accreditation of ISO 15189, managerial and technical requirements, both have various elements that should be taken care of to obtain the accreditation smoothly. It has been observed that the major challenge that the laboratories have been facing in the process is the skilled and responsible staff to execute the operation efficiently. Most of the time, laboratories fail to provide the environment healthy services as the waste product of the medical laboratories should be disposed of responsibly as it contains various bacteria which may cause serious diseases among the people who would have encountered ?? with those bacteria. Another study stated that there are referral issues as it has been observed that it is quite difficult for any laboratory to provide all of the services on their own; therefore, they would be referring patients to other laboratories for any specific test. In such a case, when the laboratories are referring the patient sample, they must consider that the lab where they have been referring the patient should also have the accreditation of ISO 1519.
Sometimes, there is an issue with the lab documentation; it has been observed that medical labs usually fail to maintain a record of the complaints and the corrective measures they have taken before. During the application for accreditation of ISO 15189, the lab must maintain a record of their complaints, and they should take corrective measures, which are to be documented. Moreover, the preventive measures that the lab has been taking should also be documented; otherwise, the lab would not be able to obtain the accreditation of ISO 15189. Another study conducted by Tembuyser et al. (2016) shows that issues have been reported regarding the accreditation of ISO, such that labs usually avoid internal audits or there is a discrepancy in the lab records. Moreover, it has also been observed that the challenges that the lab has been facing for the accreditation of ISO are the financial constraints, as it has been observed that ISO requires that the equipment of the lab should be updated. The staff operating it should be skilled to use the equipment, meaning they would be paying higher salaries. Collectively, these problems and challenges make the process of accreditation of ISO 15189 more difficult for the labs. Good.
Effective Strategies to Sustain the Challenges of Accreditation of ISO 15189
It has been observed that the labs usually face two types of issues for the accreditation of ISO 15189; the first is financial constraints and other managerial issues. However, it depends on the lab's situation and how much finance they can arrange for their operation. However, the labs can resolve their managerial issues and cope with accreditation challenges. It has been observed that when there is a requirement for skilled and competent staff, companies suggest training their former staff instead of hiring new ones (Hillary and Balu, 2018). It has been observed that when the organization has been providing training for their former staff instead of hiring new ones, they feel more valued by their organization and try to work efficiently. It would be easier for the organization to ask them for their contribution, to which they would have to go out of the box. Before submitting your final dissertation, ensure your in-text citations align with your list of references at the end. What does this mean? It means that each citation should point to a listed reference, and each listed reference should have at least one citation pointing to it. From an academic point of view, this is quite important.
The lab managers should be focused on the documentation process, and each nominal detail should be documented, and periodic maintenance and quality checks should be conducted. They should also focus on the lab's internal audit and report generation process. Moreover, the lab managers should also evaluate the gaps for improvement before applying for accreditation and try to fix all the issues once everything has been resolved; they should then apply for it.
Consistent spacing
Theoretical Framework <- is good.
It has been observed that medical laboratories have been facing managerial issues in getting accredited with ISO 15189. Therefore, the principles of administrative management by Henri Fayol are the most appropriate principles for the issue. (Peaucelle and Guthrie, 2015). It has been observed that the principles of administrative management by Henri Fayol provide five steps that the managers of any organization should follow to rectify the issues that they have been facing. The first step identified by Fayol is planning; it has been observed that planning before any project is one of the necessary aspects to consider. Later, organizing the necessary arrangements is the most important element, as it has been observed that the execution of any plan is impossible without efficient organization (Fayol, 2016). After arranging the necessities, commanding, coordinating, and controlling are the things that never end for managers. Following the principles of administrative management by Henri Fayol, obtaining accreditation for the managers of the medical laboratories would be easier. Are you sure about using Fayol? Of course, he’s a very famous management writer – one of the seminal theorists – but his work was done long ago.
In addition to the principles of administrative management, managers of medical laboratories should also use contingency theory as it helps them deal with their employees and the technology they have been using. It has been observed that there is a need for technological updates in the requirement of ISO 15189, and the medical laboratories, therefore, have to manage their workforce accordingly. This theory helps managers to deal with their employees and bring the element of leadership to all levels of their organization (Otley, 2016). Therefore, the incorporation of contingency theory is one of the best theories that can help them cope with the issues of medical laboratories and fulfil the requirement of ISO 15189. So, are you saying these theories will influence your research? If so, how? Do you expect these theories to influence your data gathering and how you organize the discussion of findings?
Whether “theoretical framework” and “conceptual framework” mean the same is contested, but the arguments are difficult to follow. I recommend that, at this level, you assume they *are* the same. If not, you will open a whole can of worms. So I think you should have one or the other. I’ve been doing this for 10 years, and you’re the first-ever student I’ve supervised who’s had both.
Conceptual Framework
A conceptual framework serves as the intellectual scaffolding of a study, providing a structured lens through which researchers conceptualize, organize, and interpret their findings. The architectural blueprint shapes the study's design, guiding the researcher in constructing a coherent and comprehensive understanding of the phenomena under investigation. Like an intricate map, a conceptual framework charts the relationships between variables, offering clarity in navigating the complex terrain of research and unveiling the underlying structure that binds diverse elements together into a unified whole.
This is nice and clear. What about a caption?
Factors identified affecting quality and competence in medical laboratories impact it on a higher level. The independent factors identified are poor staff motivation, lack of knowledge and appropriate skills, non-calibrated and non-updated equipment, and lack of management support. In contrast, the dependent factor is quality and competence in medical laboratories. Poor staff motivation is significantly related to quality and competence, leading to decreased quality and competence in a clinical setting. Lack of knowledge and appropriate skills are also significantly associated with quality and competence in medical laboratories, which have a higher impact on the quality and competence, showing that quality and competence decrease with lack of knowledge and appropriate skills. Non-calibrated and non-updated equipment also directly relates to quality and competence in medical laboratories, which has a higher impact on reducing quality and competence in performing functions in a clinical setting. Lastly, lack of management support is significantly related to quality and competence in medical laboratories, with the highest impact on the entire clinical setting. It may lead to highly negative outcomes and a lack of efficiency and effectiveness in medical laboratories' service quality and competence. Very good
Conclusion
This chapter of the study provides a detailed literature review regarding the issues and challenges that medical laboratories have been facing for the accreditation of ISO 15189. Can you think of a clearer way to write that? In addition, the significance and the impact of ISO 15189 have also been discussed, along with the factors affecting the quality and competence of medical laboratories. Furthermore, the role of ISO has also been studied, and the management theory has been identified as the most appropriate theory regarding the issues in the medical laboratory. In the last section of the chapter, a conceptual framework has also been provided showing the relation of the identified factors to the quality and competence of the medical laboratory. Good.
It's a very good draft chapter. I didn’t see much criticism, though.
Shane
References
Alizadeh, S. and Chavan, M., 2016. Cultural competence dimensions and outcomes: a systematic review of the literature. Health & social care in the community, 24(6), pp.e117-e130.
Amato, F., López, A., Peña-Méndez, E.M., Vaňhara, P., Hampl, A. and Havel, J., 2013. Artificial neural networks in medical diagnosis.
Antonelli, G., Padoan, A., Aita, A., Sciacovelli, L. and Plebani, M., 2017. Verification of examination procedures in clinical laboratory for imprecision, trueness and diagnostic accuracy according to ISO 15189: 2012: a pragmatic approach. Clinical Chemistry and Laboratory Medicine (CCLM), 55(10), pp.1501-1508.
Beastall, G., Kenny, D., Laitinen, P. and ten Kate, J., 2005. A guide to defining the competence required of a consultant in clinical chemistry and laboratory medicine. Clinical Chemistry and Laboratory Medicine (CCLM), 43(6), pp.654-659.
Berendes, S., Heywood, P., Oliver, S. and Garner, P., 2011. Quality of private and public ambulatory health care in low and middle-income countries: systematic review of comparative studies. PLoS medicine, 8(4), p.e1000433.
Bishop, M.L., Fody, E.P. and Schoeff, L.E. eds., 2013. Clinical chemistry: principles, techniques, and correlations. Lippincott Williams & Wilkins.
Brown, C.A. and Busman, M., 2003. Expatriate health-care workers and maintenance of standards of practice factors affecting service delivery in Saudi Arabia. International Journal of health care Quality Assurance, 16(7), pp.347-353.
Büyüközkan, G., Çifçi, G. and Güleryüz, S., 2011. Strategic analysis of healthcare service quality using fuzzy AHP methodology. Expert systems with applications, 38(8), pp.9407-9424.
Chahal, H. and Bala, M., 2012. Significant components of service brand equity in the healthcare sector. International Journal of health care quality assurance, 25(4), pp.343-362.
Chapman, S.A. and Himmerick, K., 2017. California’s Medical Laboratory Technician (MLT) Workforce: Opportunities and Key Policy Issues.
Chhea, C., Warren, N. and Manderson, L., 2010. Health worker effectiveness and retention in rural Cambodia. Rural Remote Health, 10(3), p.1391.
Das, J., 2011. The quality of medical care in low-income countries: from providers to markets. PLoS medicine, 8(4), p.e1000432.
Fayol, H., 2016. General and industrial management. Ravenio Books.
Ferrari, C.B., Andrade, M.A.B., Adamowski, J.C. and Guirro, R.R.J., 2010. Evaluation of therapeutic ultrasound equipment performance. Ultrasonics, 50(7), pp.704-709.
Gargis, A.S., Kalman, L., Berry, M.W., Bick, D.P., Dimmock, D.P., Hambuch, T., Lu, F., Lyon, E., Voelkerding, K.V., Zehnbauer, B.A. and Agarwala, R., 2012. Assuring the quality of next-generation sequencing in clinical laboratory practice. Nature Biotechnology, 30(11), p.1033.
Harmening, D.M., 2018. Modern blood banking & transfusion practices. FA Davis.
Hillary, N. and Balu, L., 2018. The Effect of Employee Relation on Employee Morale at St. John’s Medical Hospital, Bengaluru.
Houghton, C.E., Casey, D., Shaw, D. and Murphy, K., 2012. Staff and students' perceptions and experiences of teaching and assessment in Clinical Skills Laboratories: Interview findings from a multiple case study. Nurse Education Today, 32(6), pp.e29-e34.
Jones, G.R. and Jackson, C., 2016. The Joint Committee for Traceability in Laboratory Medicine (JCTLM)–its history and operation. Clinica Chimica Acta, 453, pp.86-94.
Marinucci, F., Majigo, M., Wattleworth, M., Paterniti, A.D., Hossain, M.B. and Redfield, R., 2013. Factors affecting job satisfaction and retention of medical laboratory professionals in seven countries of Sub-Saharan Africa. Human resources for health, 11(1), p.38.
McGinnis, M.R., 2012. Laboratory handbook of medical mycology. Elsevier.
Misganaw, A.S., 2016. A journey to accreditation: is ISO 15189 laboratory accreditation possible in Ethiopia? The Pan African Medical Journal, 24.
Morgan, R., Ensor, T. and Waters, H., 2016. Performance of private sector health care: implications for universal health coverage. The Lancet, 388(10044), pp.606-612.
Mosadeghrad, A.M., 2012. A conceptual framework for quality of care. Materia socio-medica, 24(4), p.251.
Mosadeghrad, A.M., 2014. Factors influencing healthcare service quality. International journal of health policy and management, 3(2), p.77.
Nasser, M., M’hamed, T., Badrane, N., Soulaymani-Bencheikh, R. and Bouazzi, O.E., 2018. Preparation for the Accreditation According to ISO 15189: A Quality Folder Realization. Int J Anal Bioanal Tech: AABST-104. DOI, 10.
Neubauer, T. and Heurix, J., 2011. A methodology for the pseudonymization of medical data. International journal of medical informatics, 80(3), pp.190-204.
Olmsted, S.S., Moore, M., Meili, R.C., Duber, H.C., Wasserman, J., Sama, P., Mundell, B. and Hilborne, L.H., 2010. Strengthening laboratory systems in resource-limited settings. American Journal of Clinical Pathology, 134(3), pp.374-380.
Oosterhuis, W.P., Bayat, H., Armbruster, D., Coskun, A., Freeman, K.P., Kallner, A., Koch, D., Mackenzie, F., Migliarino, G., Orth, M. and Sandberg, S., 2018. The use of error and uncertainty methods in the medical laboratory. Clinical Chemistry and Laboratory Medicine (CCLM), 56(2), pp.209-219.
Otley, D., 2016. The contingency theory of management accounting and control: 1980–2014. Management accounting research, 31, pp.45-62.
Peaucelle, J.L. and Guthrie, C., 2015. Henri Fayol. In The Oxford Handbook of Management Theorists.
Plebani, M., 2012. Quality indicators to detect pre-analytical errors in laboratory testing. The Clinical Biochemist Reviews, 33(3), p.85.
Plebani, M., Sciacovelli, L., Chiozza, M.L. and Panteghini, M., 2015. Once upon a time: a tale of ISO 15189 accreditation. Clinical Chemistry and Laboratory Medicine (CCLM), 53(8), pp.1127-1129.
Plebani, M., Sciacovelli, L., Marinova, M., Marcuccitti, J. and Chiozza, M.L., 2013. Quality indicators in laboratory medicine: a fundamental tool for quality and patient safety. Clinical biochemistry, 46(13-14), pp.1170-1174.
Rosenzweig, M., Giblin, J., Mickle, M., Morse, A., Sheehy, P., Sommer, V. and Bridging the Gap Working Group, 2012, March. Bridging the gap: A descriptive study of knowledge and skill needs in the first year of oncology nurse practitioner practice. In Oncology nursing forum (Vol. 39, No. 2, p. 195). NIH Public Access.
Shieh, J.I., Wu, H.H. and Huang, K.K., 2010. A DEMATEL method in identifying key success factors of hospital service quality. Knowledge-Based Systems, 23(3), pp.277-282.
Sikaris, K.A., 2017. Enhancing the clinical value of medical laboratory testing. The Clinical Biochemist Reviews, 38(3), p.107.
Talib, F., Rahman, Z. and Azam, M., 2011. Best practices of total quality management implementation in health care settings. Health Marketing Quarterly, 28(3), pp.232-252.
Tembuyser, L., Van Campenhout, C., Blanckaert, N. and Dequeker, E.M., 2016. ISO 15189-accredited laboratories fulfil the JCI Hospital Accreditation Standard requirements for the use of referral laboratories: report of a consensus meeting. Accreditation and Quality Assurance, 21(6), pp.425-431.
Get 3+ Free Dissertation Topics within 24 hours?