
Leading Change Management in Business
January 19, 2022
The Abuse of Corporate Veil: A Comparative Analysis of Corporate Veil Lifting Approaches in UK and US laws
January 28, 2022Abstract
Rocket launches have the potential to impact the atmosphere in manners that are immediate, episodic, and even long-term. Research shows that the stratosphere is immediately affected after the launch of a rocket vehicle. The impact spreads on the trajectory of the vehicle along its flight path. It is evident that emissions from certain types of launch vehicles like solid rocket motors (SRM) significantly agitate the atmosphere along the launch trajectory. The current report details how ozone concentration is temporarily reduced with the production of an aerosol plume and its consequent combustion products which includes chlorinated compounds, alumina, NOx, and other reactive radicals. The report shows that there is a great potential for rocket fuel to impact the global and local stratospheric ozone due to the increase in chlorine. The report focuses on current research that has contributed to the literature on ozone depletion from rocket fuel. In addition, the report presents a proposal to further investigate depletion based on the introduction of alternative rocket fuel.
Background and History
The fascination for space exploration by man has been ongoing since the 1940s, with German scientists testing the V-2 rocket successfully in 1942. Since then both the then Soviet Union (current-day Russia) and the United States of America have conducted space programs beginning in the late 1950s using various launch vehicles. In itself, rocket launches used for space exploration are gaining in popularity due to advancements in technology. A greater number of rocket launches are being demanded space exploration, national security, reduction in launch costs and the emergence of private markets for tourism, solar energy, and manufacturing. Historically, strategic missiles had used various liquid-propellant engines starting with the German V-2 rocket. However, there was an increased need for instant readiness of strategic missiles which required for a different type of fuel to be used, eventually leading to the use of various solid propellants.
Rocket launches are seen to affect the natural environment specifically its impact on changing the composition of the atmosphere during its flight path. According to Ross, Toohey, Rawlins, Richard, Kelly,…Sheldon (2000) about forty per cent of the total ozone present is destroyed from rocket plumes. Early research conducted by Potter (1977), Potter (1978), Prather (1990), and Bennett and Hinshaw (1992) conclude that solid-fuel rocket motors used in large space launch vehicles release gases and particles that may considerably impact the stratospheric ozone densities that are along with the play of the vehicle. Both liquid and solid rocket propellants deplete the ozone in different ways. The method of ozone depletion in the stratosphere by solid rocket exhaust products is caused by it containing large amounts of chlorine substances.
Effects of Solid Rocket Fuel
According to Ross, Danilin, Weisentein, and Ko (2004) solid rocket motors are responsible for about one-third of the stratosphere propellants in the atmosphere from rockets while liquid propellants account for two-thirds. Terry, Sippel, Pfeil, Gunduz, and Son, (2016) reported that solid rocket fuel is made up of aluminium, ammonium perchlorate, and a polymer matrix. It is the combustion of this solid fuel that results in the chlorine in the exhaust that is derived from ammonium perchlorate. The main volatile compound that is created from the combustion of solid rocket motors from its plumes is hydrochloric acid (HCl). The major presence of chlorine in rocket plumes is attributed to HCl, which is derived from the process of afterburning that allows for the conversion of HCl to pure chlorine molecules. Ross et al. (2004) describe afterburning as an occurrence from rocket engines burning propellants with an excess of fuel compared to the oxidizer. It is noted by the previous research that conversion between the two takes place from the high concentration of oxygen (O) and hydrogen (H) molecules (M. N. Ross et al., 2004). Outlined in the research is a possible reaction for the conversion:
![]()
Equation 1‑1: Conversion Reaction in Afterburning (Source: Ross et al., 2004)
Research conducted by Bloss, Nickolaisen, Salawitch, Friedl, and Sander (2001) found that the catalytic ozone destruction cycle involves ClO self-reaction that is considered as the dominant gas-phase ozone layer destruction process that mostly takes place in the stratosphere. Bloss et al. (2001) outline the following mechanism as the main cause of ozone destruction:
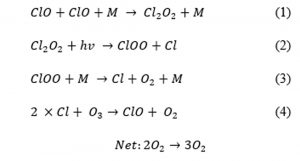
Equation 1‑2: Gas-Phase Ozone Destruction Process (Bloss et al. 2001)
This and other chlorine mechanisms show that Cl2 combines with ozone once it is exposed to light from the hypochlorite (ClO) molecule and diatomic oxygen (O2). Resulting in the impact of oxygen available to inhale. Then a ClO combines with another ClO in the presence of a third body (M) which is described by Bloss et al. (2001) as being a solid surface that allows the entire reaction to take place. Lohn, Wong, Spencer, Meads, and Molina (1996) found that the reason this entire reaction is so harmful to the ozone is that chlorine is able to recycle and destroy another ozone molecule over and over again in a continuous cycle.
Researchers have found that on average, over five to ten molecules in the ozone are expended by every chlorine atom that is deposited from the rocket fuel (Bloss et al., 2001; Lohn et al., 1996; Martin Ross, Darin Toohey, Manfred Peinemann, & Patrick Ross, 2009; M. Ross et al., 1997; Toohey, 2003). Ross et al. (1997) found that chlorine is confirmed to be the greatest contributor to ozone loss from rocket fuels based on the data collected from rocket launches, this results from the need for sunlight to activate the Cl in order to begin the destruction of the ozone cycle by these molecules.
Effects of Liquid Rocket Fuel
Aside from solid rocket fuels, liquid rocket fuels are also used in rocket launches. Liquid rocket fuel is considered to be better in use as the rocket thrust can be adjusted while in mid-flight compared to solid rocket fuel that burns at a fixed rate without any way to alter it. Many in the scientific community believe that combustion from liquid rocket fuel causes some destruction of the ozone by its nitrogen mechanism. Ross et al. (2004) expressed that nitric oxide (NO) is a known mechanism that allows the destruction of the ozone and was once present from the combustion of liquid rocket fuels which has mostly been eliminated.
Later, Ross and Sheaffer (2014) reported that no mechanism is not as ozone-depleting as solid rocket fuel. The destruction of the ozone was short and only occurred immediately after launch which reduces the time for ozone destruction. The NO mechanism is known to have a slower catalytic cycle than compared to ClO mechanism leading many to conclude that liquid rocket engines are less harmful to the stratospheric ozone than their solid counterparts (Ross M. N. et al., 2000; M. N. Ross et al., 2004; Terry et al., 2016).
The Modern Paradigm
Currently, rockets are seen to have a negligible impact on the destruction of the ozone layer on a global scale compared to other causes. However, there is renewed interest on a global scale for rocket launches which may begin to have a larger impact on the ozone in the future. Therefore, it becomes imperative to consider changes in fuel sources to decrease the effects that rocket fuel can have. Lohn et al. (1996) noted that advancements have been made to make liquid nitrogen fuel non-nitrogen based in order to decrease the impact of NO on ozone loss.
Zittel (1994) had suggested using chlorine-free rocket fuel in order to eliminate the impact of chlorine radicals on destroying the ozone. Limited research is available to predict the extent of this destruction but studies have been conducted to understand its significance with limited data. Smith, Edwards, and Pilson (1999) argued rocket launches have the potential to impact the atmosphere in an episodic manner (immediate impact), and a cumulative manner (long-term impact). One of the immediate effects found on the stratosphere is after launching with the destruction of the ozone being caused along or near the vehicle’s flight trajectory.
There are certain types of launch vehicles that are seen to significantly disturb the atmosphere along the trajectory with an estimated range of 10km or less from the vehicle’s passage (Smith et al., 1999). During this time, the ozone concentration is momentarily reduced allowing for aerosol plumes to be produced along with combustion products like chlorinated compound, alumina, NOx, and reactive radicals; these are known to change the actual chemistry along the path trajectory (Smith et al., 1999).
There is also a known local effect on the stratosphere by rocket launches that reduces the ozone extensively for up to two hours through the expanding exhaust plumes after the rocket’s launch (Smith et al., 1999). Within the expanding rocket plume, an ozone hole is formed and continues to increase in size through the two hours after launch. However, researchers have found that during this time the ozone concentrations recover to their original levels pre-launch as time passes with the ozone back-filling into the hole through diffusive processes. This phenomenon depends on factors like the number of emissions that are released and the size of the launch vehicle. It has been recorded though that the time for the hole to refill to its normal ozone levels was 3000 seconds at 15-20km and 6000 seconds at 40km, these numerical are based on the measurements concluded by Ross et al. (1997) and the modelling found in studies of Lohn et al. (1999).
For some time now, researchers believed that a relatively inactive form of Cl, hydrogen chloride (HCl), was the only solid rocket motor (SRM) chlorine emissions species. Further researcher into the radicals with more weighted calculations and laboratory experiments such as () have concluded that chlorine is present also as Cl2 or Cl radicals. It may be considered as minor for some, but it is significant in that while HCl mainly adds to the worldwide chlorine burden causing global ozone depletion, the extremely active forms of chlorine do participate in the destruction of ozone at an immediate and local level.
In terms of rocket launches, the process of depletion in the ozone is controlled through the rate at which the particular species of molecules formed through rocket plume diffuse into the ambient atmosphere, the reaction that Cl and its product of hypochlorite with ozone, and the successive reproduction of Cl through photoreaction and others. Researchers have developed model simulations of excessive ozone losses within the first few hours of rocket launch which are documented through the launch data of vehicles like Space Shuttle, Titan III, and Titan IV (Lohn et al., 1996; M. Ross et al., 1997).
Moreover, to the aforementioned effects on local ozone, the emissions from rockets are thought to have long term and global effects on the stratospheric ozone. Many researchers believe that the potential long term and global ramifications are attributed to the long lifespans that are characteristic of alumina particulate and HCl in the stratosphere. Ko (1999) does argue that rocket motor emissions only represent a small fraction of the entire manmade impact on the chemistry of the stratosphere.
Studies like Jackman et al. (1998) had carried out detailed modelling calculations of ozone depletion in the stratosphere using the launch rate of nine ‘Space Shuttle’ launches, while Molina (1999) tested three from the Titan IV launches annually through the use of reaction probability measurements of ClONO2 with HCl on alumina surfaces. Both studies’ results indicate that the impact on the annual global average of total ozone decreased by 0.025% in 1997. Based on these results one-third of the decrease came about from solid rocket motor emitted alumina while the remaining portion resulted from solid rock motor emitted HCl. The results produced by Jackman et al (1998) and Molina (1999) were substantiated independently from the research of Lohn et al. (1999) and Ko et al. (1999).
Smith et al. (1999) find that some of the potential long-term effects from using solid rocket motors include a worldwide reduction in the stratospheric ozone, with increasing Cl load in the stratosphere, and an increase in the specific particulate burden. Based on the modelling presented by Jackman et al. (1998), Ko et al. (1999), Lohn et al. (1999), and Molina (1999) it is evident that there are global ramifications from SRM propellants but they are very minor, but existent and long-term.
Major Issues Explored
A majority of studies examining the impact of rocket fuel on the ozone often time uses either in-situ measurements or laboratory studies to conduct their research. Research conducted by Ross et al. (1997) is an example of an in-situ study. A majority of in-situ measurements suggest that solid rocket motor launches create ozone loss immediately after launch. When comparing the available in–situ data to that of modelling attempts, it is evident that models produce results that show a slight underestimation of the size and duration of the area in which ozone is removed by both large and medium launch vehicles. Even though such a loss occurs, the reduction in the ozone only exists over a few kilometres of area and was later on found to be much smaller than expected. The local ozone reductions had decreased to a value near zero over the course of a few days, in which the effects to the concerned region also reduced and became smaller than a TOMS satellite is able to detect and log.
Laboratory studies conducted by Disselkemp (1999) focused on the uptake and deposition of NO and NO2 on the surface of Al2O3, due to the adverse effects of both compounds on the environment, out of which, one effect is increased catalytic destruction of the ozone by halogen species and derivatives. Consider that the concentration of the nitrogenous oxides is 10 parts per billion volume then 640 particles/cm3 (2.5 x 10 10 molecules/ cm3) of Al2O3 is required to remove all particles of the nitrogenous oxide. However, the volume of aluminium oxide in the exhaust gases is far too low to cause a significant amount of damage to the ozone as compared to the damaging effects of halogen species, specifically chlorine gas.
Another ramification of this type of chemical activity was to consider the uptake of halogens on the surface of aluminium oxide. Here, Disselkemp (1999) proposed that the absorbing of halogens by the aluminium oxide particles to evolve oxides of nitrogen have the ability to increase the concentration of ozone. This can be done as the concentration of halogen species is greatly reduced and hence, a decrease in the catalytic destruction of the ozone is also observed, however, no additional data is available to support this idea. As a result, further study is required in order to understand and characterize the chemistry of halogen species.
Molina (1999) added to the plethora of research using laboratory studies by analysing the reaction probability (γ) of ClONO2 with HCl on an alumina surface. Molina (1999) concluded that the probability of the reaction between the two was a value that was γ=0.02 under conditions similar to that of the lower stratosphere. This reaction was categorized as a near-zero reaction in HCl due to the dependency of the reaction on the presence of absorbed water layers and not on the nature of the refracting oxide surface itself. Detailed studies showed that a noteworthy fraction of the injected alumina surface area is catalytically active and remains un-affected in the stratosphere by sulfuric acid vapours.
It took about eight months for the sample of the alumina surface to get covered by sulfuric acid in a single layer with an accommodation coefficient of 0.1. Finally, coalescence with sulfuric acid vapours in the stratosphere is insignificant for alumina particles with a diameter larger than 1μm before being removed from the stratosphere. For alumina particle distributions less the 0.13μm, the mass-weighted atmospheric lifetime is approximately 0.3 years as the reactivity of such particles is extremely small, regardless of sedimentation and collision removal status. Ko et al. (1999) replicated and produced the same results from their research’s 3-D model.
Critical Analysis
Methodologies used in analyzing ozone depletion impact further research need and policies that are created to minimize the stratospheric ozone depletion from rocket launches. As discussed in the previous section, depletion of local stratosphere ozone within the plume of the launch vehicle is measured using in-situ measurements and other field techniques that can only be used in a small window of opportunity. When examining the phenomenon on a worldwide scale, depletion of the ozone from rockets is calculated primarily through the use of theoretical models. However, these models are known to produce detection limits that are below what current measurement techniques have confirmed. With the available data it is concluded that if the frequency of rocket launches that are using solid rocket motors/propellants increase from a global increase in government and private rocket launches, there will be a significant increase to the extent of the depletion of the ozone.
Due to the potential implications of using SRM rockets, it has become imperative to introduce and experiment with ways to limit pollution from substances like HCl in rocket launches in order to significantly minimize or eliminate launch site contamination leading to ozone depletion. There are many published literature available that analyzes and discusses the impact made on the ozone through ozone reactive compounds seeped into the stratosphere by SRMs such as studies conducted by Brady et al. (1994), Denison et al. (1994), Jackman et al. (1998) Ko et al. (1999), Prather et al. (1994), Ross et al. (1998), Takenaka and Yamagokoro (2004), Terry et al. (2016), Voigt et al. (2013), Zittel et al. (1994). Based on the results of these studies, Cl and oxides of Cl are present within the exhaust of SRM. These studies also concur that there are two other classes of compounds generally present in exhaust from a rocket that is known to cause ozone destruction – nitrogen oxides and hydrogen oxides. Both of these are generally present in the exhaust of every launch vehicle regardless of its size. Takenaka and Yamagokoro (2004) also found that there are species of alumina and soot from the kerosene fuel in rocket exhaust that enables heterogeneous reactions between chlorine compounds and ozone.
In order to do so, it is common for experiments that computer models of implications of rocket exhaust on the atmosphere be conducted and then validated through laboratory experiments. A majority of the data in terms of chemical (chlorine and alumina) deposits into the stratosphere are provided through the research of Bauer and Zondervan associated with The Aerospace Corporation (as cited in Brady et al., 1994). The method used for this was the total mass of exhaust being calculated through the corporation’s simulation code and then the amount of chlorine and alumina are calculated from their known percentages from the exhaust. The data is presented in tons as illustrated below.
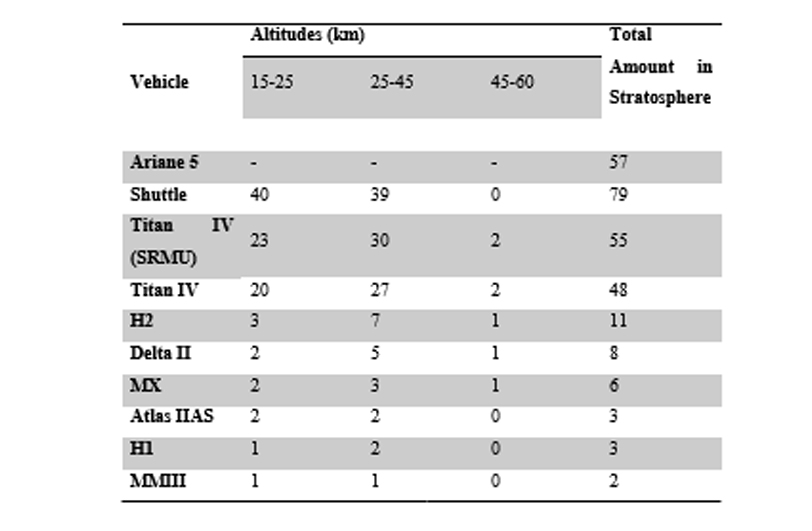
Table 4‑1: Chlorine present in Stratosphere, tons/launch (Source; Smith et al., 1999)
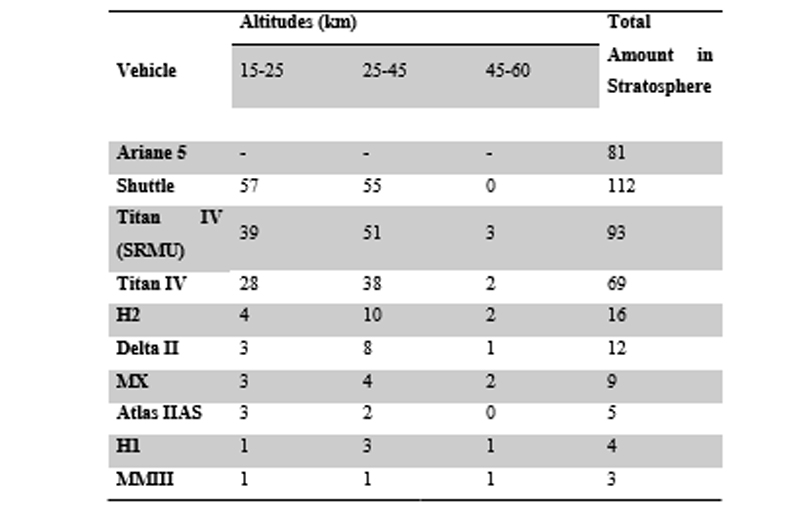
Table 4‑2- Alumina present in Stratosphere, tons/launch (Source; Smith et al., 1999)
The particles of alumina and chlorine have the potential to destroy the ozone directly as corroborated by the results in Brady et al. (1997) as these particles also contain iron and chlorine from the exhaust which is known to be more reactive. It is evident from the data accumulated in tables 2-1 and 2-2 that individual space launches release a small portion of the total potential ozone reactive species loading in the stratosphere. Nonetheless, the accumulative effect of the rocket launches globally mare is even more significant as asserted by Lohn et al. (1999), Takenaka and Yamagokoro (2004) and later Voigt et al. (2013).
When analyzing the various literature available, the study of Molina et al. (1999) stands out as it conducted laboratory experiments on chemical processes involving the effects of particles emitted from SRMs into the stratospheric ozone. The study places emphasis on the proficiency of the catalytic chlorine activation process that is known to take place on the Al2O3 particle surface. The proposed reaction from previous work in Molina et al. (1996) presented the catalyzed reaction using α–alumina surfaces.
![]()
Equation 4‑1: Catalytic Chlorine Activation Process (Molina et al., 1999)
According to Smith et al. (1999), the reaction represents the most important process that results in the transformation of reserved Cl species to free Cl atoms in the stratosphere. Studies such as Ko et al. (1999) has shown that these atoms efficiently deplete the ozone through its catalytic cycles. Based on the laboratory experiments Molina (1999) conducted the hypothesis made was that water was absorbed on the surface of the alumina particles allowing the reaction of activation Cl as it was made available a portion of high-affinity hydrochloric acid molecules. The study infers that alumina particles emitted by solid rocket motors were catalytically active in the stratosphere as they were covered in absorbed water. The experiments also found that the particles recovered their surface OH groups by having a reaction with water vapour in the form of OH and HO2 radicals.
Building on the research it was essential to understand the immediate effect of exhaust plumes in the atmosphere. However, Smith et al. (1999) report that there is a complete lack of data on the makeup of exhaust plumes during the first several hours immediately after launch which makes it difficult to develop models on the depletion of the ozone. Eventually, the first ozone measurement on solid rocket motors plume was reported by Pergamet et al. (1997). According to Pergamet et al. (1997), there was a 40 per cent reduction during a single plume passed the altitude of 18 km. The study did suggest that it observed a loss that was caused by the presence of an ozone destructive exhaust component. Unfortunately, the measurement of uncertain reliability was not repeated in the in-situ investigations. It was left unclear if the predictions of substantial ozone loss in solid rocket motors plume were valid.
McDonald et al. (1995) built on the works of Molina (1999) and Pergamet et al. (1997) in order to show the impact of ozone destruction due to the various principle classes of ozone destroyers. Based on the data that is illustrated in the table below, the portion of the ramifications identified associated with rocket launches was less than 0.034 per cent.
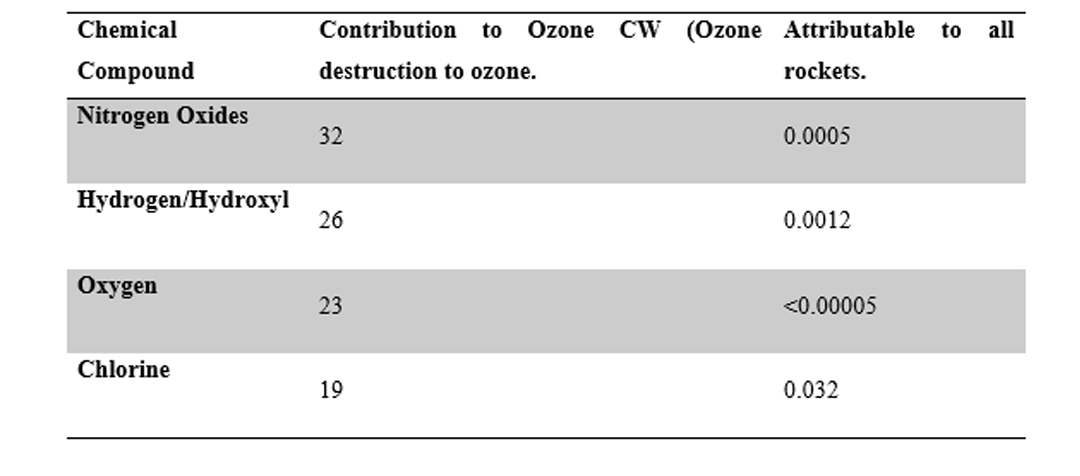
Table 4‑3- Chemical Compounds in exhaust causing ozone Destruction (McDonald, 19991)
The data also illustrates that Cl has the most impact from rocket emission around the world using McDonald et al. (1995) outcomes. Vehicles like the Zenit-3SL are known not to release Cl and its compounds, and therefore it was concluded by FEAFSLP (1999) that the Sea Launch Programme would not have a significant impact on the worldwide ozone. It should be noted that the in-situ methods from McDonald et al. (1995) were replicated by Tishin et al (1995) to analyze Russian made rocket vehicles which also led to the same conclusion presented by McDonald et al. (1995).
Other studies like Brady and Martin (1995) and Brady et al. (1997) had estimated the potential ozone hole in terms of size and duration from the launching of vehicles V 551/552 and Delta Iv M+. The use of in-situ for these studies including Brady et al. (1994) was to compare the local stratosphere impacts from vehicle launch. Their data is illustrated in the table below.
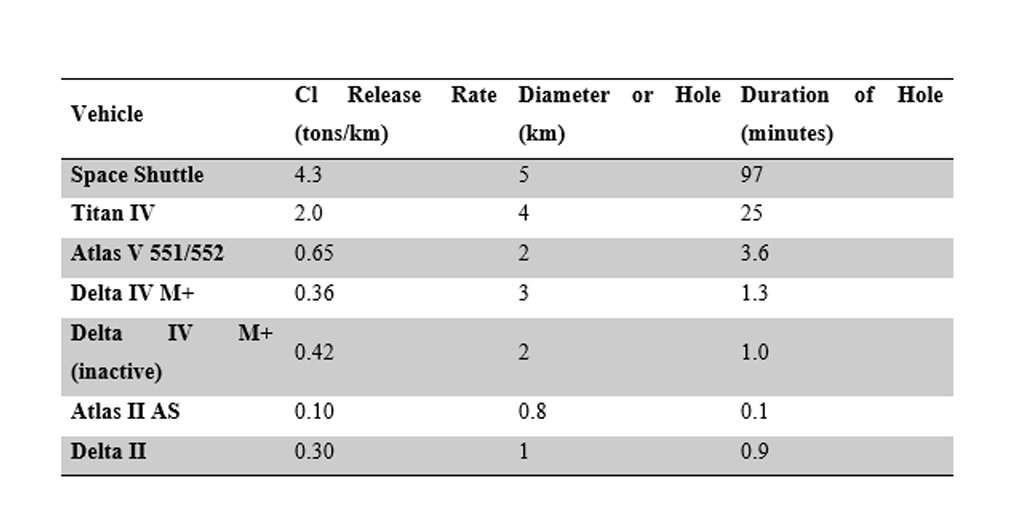
Based on the values for each of these studies, the lift vehicle that is considered proposed needs to have an estimated ozone hole that lasts for just a few minutes and has a limited size in terms of its ozone hole. In all three studies, it is concluded that Space Shuttle has the most Cl release rate compared to other launch vehicles in addition to having a greater hole diameter and distance.
Table 4‑4: Comparison of Lift Vehicles with Solid Rocket Motors (Brady et al., 1994; Brady and Martin 1995, Brady et al., 1997)
Proposal
Many previous studies into the improvement of rocket fuel have looked at ways to eliminate the production of Cl, HCL, AL2O3, and CO2. By proposing alternative propellants there is a greater chance that ozone depletion is reduced to a significant or completely. But there is also a chance that the same effects can come about but through the use of bio-mass to make bio-fuel for rockets. Researchers at Georgie Institute of Technology and Joint BioEnergy Institute were able to genetically engineer a specific version of E. Coli that is capable of producing pinene (Sarria, Wong, Martín, Keasling, & Peralta-Yahya, 2014). Keeping to this theme of bio-mass the following is proposed.
Introduction
Biomass is a comparatively inexpensive material even to plastic as it is derived from living or decomposed organisms. Biomass can be converted into biofuel which is achieved through various methods. Historically speaking, humans have cultivated and harnessed biomass energy since the time that Neanderthals discovered wood can make fire. Today’s economy is demanding a clean, original, sustainable, and renewable resource that minimizes damage to health and the surrounding ecosystem. Thus, many countries are shifting to a more renewable energy-based economy as it is having the potential for long term sustainable fuel that can meet the demands of a growing economy without depleting natural resources. Hydrocarbons are advantageous since they can be used as fuels, lubricants, and solvents without the impact of environmental risks such as ozone depletion, air pollution, and global warming.
The purpose is the production of hydrocarbons which can be further exploited depending on needs. Catalytic depolymerization of plastics and biomass is a method that has been widely used for extracting hydrocarbons which can be used as industrial feedstock. Currently, wastes are being used to produce many other resources, such as renewable and clean fuel.
Extracting hydrocarbons from waste products is beneficial on an economic basis as it is cheaper to find the startup material to begin the process. Furthermore, from the extraction process, there will be an accumulation of smaller molecules that can be used in the production of new petrochemical and plastics.
The process of Thermal Depolymerization (TDP) using catalytic-fast pyrolysis is used for the reduction of complex organic materials, specifically for this study; plastics and biomass. The process is known to produce products such as aromatic compounds and hydrocarbons. The energy from the pressure and heat of the process decomposes long chains of hydrogen, oxygen and carbon into shorter chains of hydrocarbons that are currently known to have a maximum length of an estimated eighteen carbons. Therefore, there is great potential to further the extraction process to produce petrochemicals
Current Literature
Catalytic Pyrolysis is known to be an emerging technology for converting biomass like lignocellulosic into liquid products such as benzene, xylene, and toluene which are known to be imperative feedstock for various industries as well as high octane liquid fuel. The specific process of catalytic fast pyrolysis involves the use of fast heating biomass feedstock in the presence of a zeolite catalyst at a temperature that is between 400°C to 700°C. This process has been extensively used for the production of aromatic from glucose through the process of catalytic fast pyrolysis (Li et al., 2013).
Catalytic pyrolysis is a developing technology being used to study biomass conversion technologies to hydrocarbon fuels. It is essential to incorporate the barriers and target research toward reducing the conversion costs of using such a technology. Many studies have analyzed process designs and preliminary economic estimates for the several pathways that are found in the process reaction. Upon further analysis from a study (Biddy et al., 2013) it was found that woody biomass using ex-situ catalytic fast pyrolysis was upgraded to using hydrocarbon blendstocks of gasoline-, diesel-, and jet- range hydrocarbons. Taken into consideration is a process block that may be used to conduct the research as it has been beneficial to many researchers before.
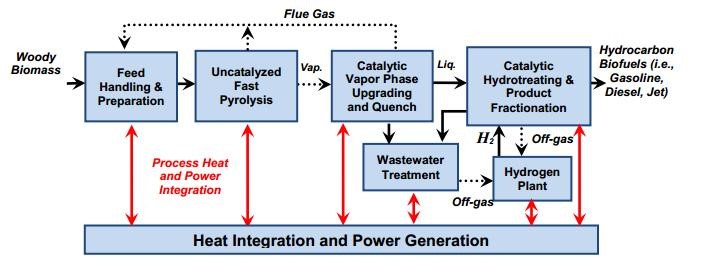
This process diagram is an outline that can be improved in terms of the development of catalysts with improved yields, stability, lifetimes, maximizing overall conversion to the desired hydrocarbon product, and developing biofuels that can contribute to the current economy. The model is based on the circulating fluidized bed that consists of a pyrolysis reactor, cyclones, and combustor (Biddy et al., 2013).
There are various advantages of using catalytic fast pyrolysis for the conversion of biomass to gasoline range aromatics as it is able to directly convert solid biomass into liquid fuel. Other routes that are known to convert solid biomass to liquid fuel in multiple steps at longer residence times result in a drastic increase in the cost of biomass processing (Carlson, 2010).
Proposed Study
In order to produce the end products of biofuel the process of Catalytic Pyrolysis will be used on biomass, plastics, and then in combination (biomass + plastics). Initial testing will be performed followed by investigations which are as follows:
- Analyze the operation of Catalytic Pyrolysis using various techniques.
- Identify a biomass
- Identify a plastic
- Identify catalyst being used
- The process beings with catalytic pyrolysis of chosen biomass, then plastic, and a combination of plastics + biomass
- Identification of products from each of the pyrolysis
- Observe the feasibility and success of the process
- Identify issues, problems in experimentation and areas in which improvement can be made.
- The conversion efficiency of biomass into products
- Affect on & Activity of catalyst
- Production Yield
- Formation & Yield of coke in processIdentification of parameters:
Feasibility
Recourse Summary
Impact Statement
Impact Summary
The continuation of the research on improving rocket fuel for limiting its adverse impact on the ozone large is a potential issue that affects people from academia to corporations to private citizens worldwide. By further researching the issue with better technology and vigour the research will first and fore mostly benefit the space launch industry in both the private and public sectors. Public sector space launch organizations are already examining the adverse impacts that launch emissions have on the ozone stratosphere.
Further research into the field as proposed will allow for applicable solutions, especially for solid rocket motors in terms of limiting their ability to emit Cl and other harmful substances from their launches. The entire research is based on the aim of ensuring that the academic findings are available for public sector launches in order to cut costs of governments funding space programmes. The private sector is also another imperative sector that needs to be targeted for the proposed research. In recent endeavours by private companies, especially that of Space X, it is essential that for-profit companies understand the environmental ramifications of launches. Therefore, the aim of the proposed research makes it necessary to provide the research to private companies for consideration. Both the public and private sector needs to regulate rocket vehicle launches in order to ensure that there are no adverse effects on the environment.
Academic Beneficiaries
The proposed research is also of benefit to other academics that are researching or looking to research the impact of rocket fuels on ozone depletion. The subject is of great interest especially due to the known effects of climate change and immediate effects seen significant holes were developed in the ozone due to anthropogenic activities. Academics understand that it is highly likely that rocket launches in the future will be a potential danger to the ozone from the increasing interest by private and public sectors. This is imperative for the academic community to propose solutions before the frequency of rocket launches exceed. Hence, the proposed research is a stepping stone when it comes to understanding the impact of rocket fuel on ozone depletion.
References
Bennett, R. R., Hinshaw, J. C., & Barnes, M. W. (1992). The effects of chemical propulsion on the environment. Acta Astronautica, 26(7), 531–541. https://doi.org/10.1016/0094-5765(92)90124-2
Bloss, W. J., Scott L. Nickolaisen, †, Ross J. Salawitch, Randall R. Friedl, and, & Sander*, S. P. (2001, November 28). Kinetics of the ClO Self-Reaction and 210 nm Absorption Cross Section of the ClO Dimer [research-article]. https://doi.org/10.1021/jp012429y.
Brady, B. B., Fournier, E. W., Martin, L. R., and Cohen, R. B. (1994). Stratospheric ozone reactive chemicals are generated by space launches worldwide. Aerospace Report No. TR-94-(4231).
Brady, B. B., Martin, L. R., Lang, V. I. (1997) Effects of launch vehicle emissions in the stratosphere. Journal of Spacecraft and Rockets, 34, 774-779.
Danilin, M. Y. (1993). Local stratospheric effects of solid-fueled rocket emissions. Annual Geophysicae, 11, 828-836.
Denison, M. R., Lamb, J. L., Bjorndahl, W. D., Wong, E. Y., & Lohn, P. D. (1994). Solid rocket exhaust in the stratosphere: Plume diffusion and chemical reactions. Journal of Spacecraft & Rockets, 31, 435-442.
FEAFSLP. (1999). Final Environmental Assessment for the SEA LAUNCH project. [report] U. S. Department of Transportation Federal Aviation Administration Office of the Associate Administrator for Commercial Space Transportation, Washington, D. C.
Jackman, C. H., Douglass, A. R., and Smeske, K. F. (1991). A simulation of the effects of the National Aerospace Place testing on the stratosphere using the two-dimensional model. [Report] Code 916, NASAJGSFC, MD.
Jackman, C. H., Considine, D. B., & Fleming, E. L. (1996). The Space Shuttle's impact on the stratosphere: An Update. Journal of Geophysics Research, 101, 12523-12529.
Jackman, C. H., Considine, D. B. & Fleming, E. L. (1998). A Global modelling study of solid rocket aluminium oxide emission effects on stratospheric ozone. Geophysics Research Letter, 35, 907-910.
Ko, M. K., Shia, R. L., Weisenstein, D., Rodriguez, J., & Sze, N. D. (1999). Global stratospheric impact of solid rocket motor launches. [report] submitted to TRW, California.
Lohn, P. D., Wong, E. Y., Spencer, D. D., Meads, R., & Molina, L. T. (1996). The Effects of Rocket Exhaust on Stratospheric Ozone: Chemistry and Diffusion.
Martin Ross, Darin Toohey, Manfred Peinemann, & Patrick Ross. (2009). Limits on the Space Launch Market Related to Stratospheric Ozone Depletion. Astropolitics, 7(1), 50–82.
Molina, M. J., Spencer, D. D., Molina, L. T., & Meads, R. F. (1996). Chlorine activation on alumna and glass surfaces. The Impacts of Rockets on the Stratosphere Symposium, Redondo Beach California.
Molina, M. J. (1999). Stratospheric effects of rocket exhaust: Heterogeneous processes. [Report] to TRW, California.
Potter, A. (1977). Proceedings of the Space Shuttle Environmental Assessment Workshop on Stratospheric Effects.
Potter, A. E. (1978). Environmental effects of the Space Shuttle. Journal of Environmental Sciences. Retrieved from https://ntrs.nasa.gov/search.jsp?R=19780044943
Prather, M. J. (1990). The space shuttle’s impact on the stratosphere. Journal of Geophysical Research: Atmospheres, 95(D11), 18583–18590. https://doi.org/10.1029/JD095iD11p18583
Ross, M., Ballenthin, J., B. Gosselin, R., F. Meads, R., F. Zittel, P., R. Benbrook, J., & R. Sheldon, W. (1997). In-situ measurement of Cl2 and O3 in a stratospheric solid rocket motor exhaust plume. Geophysical Research Letters - GEOPHYS RES LETT, 24, 1755–1758. https://doi.org/10.1029/97GL01592
Ross, M. N., Danilin, M. Y., Weisentein, D. K., & Ko, M. K. W. (2004). Ozone depletion caused by NO and H2O emissions from hydrazine-fueled rockets. Journal of Geophysical Research, 109, 1–7.
Ross M. N., Toohey D. W., Rawlins W. T., Richard E. C., Kelly K. K., Tuck A. F., … Sheldon W. R. (2000). Observation of stratospheric ozone depletion associated with Delta II rocket emissions. Geophysical Research Letters, 27(15), 2209–2212. https://doi.org/10.1029/1999GL011159
Ross Martin N., & Sheaffer Patti M. (2014). Radiative forcing is caused by rocket engine emissions. Earth’s Future, 2(4), 177–196. https://doi.org/10.1002/2013EF000160
Sarria, S., Wong, B., Martín, H. G., Keasling, J. D., & Peralta-Yahya, P. (2014). Microbial Synthesis of Pinene. ACS Synthetic Biology, 3(7), 466–475. https://doi.org/10.1021/sb4001382
Smith, T. W., Edwards, J. R., and Pilson, D. (1999). Summary of the impact of launch vehicle exhaust and deorbiting space and meteorite debris on stratospheric ozone. [Report] U. S. Air Force Space and Missile Systems Center, Environmental Management Branch.
Takenaka S., & Yamagokoro Y. (2004). Study on three-dimensional modelling of interference problem with ozone layer by solid rocket discharge. Journal of the Japan Society for Aeronautical and Space Sciences, 3, 1–10. https://doi.org/10.2322/stj.3.1
Terry, B. C., Sippel, T. R., Pfeil, M. A., Gunduz, I. E., & Son, S. F. (2016). Removing hydrochloric acid exhaust products from high-performance solid rocket propellant using aluminium-lithium alloy. Journal of Hazardous Materials, 317, 259–266. https://doi.org/10.1016/j.jhazmat.2016.05.067
Toohey, D. (2003). Real-Time Measurements of Reactive Chlorine and Carbon Dioxide in Rocket Plumes, 27.
Voigt, C., Schumann, U., Grafi, K., & Gottschaldt, K. D. (2013). IMPACT OF ROCKET EXHAUST PLUMES ON ATMOSPHERIC COMPOSITION AND CLIMATE - AN OVERVIEW. Progression in Propulsion Physics, 4, 657–670.
Zittel, P. F. (1994). Computer Model Predictions of the Local Effects of Large, Solid-Fuel Rocket Motors on Stratospheric Ozone, 23.
Get 3+ Free Dissertation Topics within 24 hours?























 Download PDF File
Download PDF File