Carbon Trading: A Solution to Global Warming?
February 27, 2021
Civil Engineering Construction- Residential Tower
February 27, 2021Introduction
Apart from the difference in the wavelength of operation, the receiver and transmitter of an FSO link in the air is very similar to the underwater link. It should be noted that there are several factors that affect the properties of the ocean water. Location, characteristics of the content present in water, location, temperature and other temporal variations such as turbulence play a key role in determining the properties of the ocean water. It is absolutely vital to firstly identify and then understand these properties in order to build an optical link. Scattering and absorption are the main causes for the loss of optical energy when navigate the link. The multipath dispersion phenomenon caused by scattering can also have huge impacts on the optical link.
Types of Water
As highlighted before, the physical properties of oceanic water depend heavily on several factors including the geographical location and the depth of water at the point of consideration. The amount of light received by water in a vertical trajectory, at any point on the sea, is further used to categorise the water. Depending on the extent of photosynthetic life evident, the topmost layer of water is usually known as the euphotic zone. The dysphotic zone is next zone below the euphotic zone and is usually found as deep as one kilometre down and therefore it is safe to say that the sun light in insufficient to support the photosynthesis process. The aphotic zone starts from the lower boundary of the euphotic zone and extends all the way down to the surface of the ocean. The fact that the sunlight does not reach this zone at all, several oceanic species take advantage of food sources that flourish under these conditions (Garrison, 1996).
When dealing with the task of building an optical link budget in sea water, the difference properties of different zones of the ocean add to the difficulties. Due to the difference in properties of water in all three zones, a system would respond differently to all three types of water zones, going from one zone to the other.
N.G Jerlove conducted a thorough research on the subject and published a comprehensive text book in 1976. Jerlove proposed that it was essential to classify the clarity of water to achieve better results. As the system in very convenient in terms of application, it is still widely used and applied across the globe. Jerlove divided various types of water in to two main categories and named them as the littoral zone (coastal waters) and the oceanic water (deep blue sea water).
The littoral zone is further divided into nine categories whereas the oceanic zone is further divided into three groups. Water in the middle of the ocean is usually very clear. These deep blue waters in the middle of the ocean tend to be cobalt blue in colour. Studies have revealed that around 10 percent of the suns light received by these waters reach at least 90 m below the sea surface. The light does not reach deeper than 15 meters in waters where large amounts of chlorophyll are present and in the Baltic sea. From our discussion, it will be fair to conclude that the reduction of light plays a critical role in construction of a link budget (Apel, 1987).
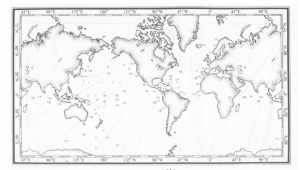
World map locating Jerlov water Types I-III (Jerlove, 1968)
From the Figure 1 above, the locations of different Jerlov types can be seen evidently. From the map above, it is possible to determine how an underwater platform might respond to different areas in the sea. Although this map changes continuously, it acts as an excellent tool to estimate for the purpose. In the next section, we will look into the scattering and absorption properties of water.
Attenuation underwater:
Attenuation underwater can be defined as the phenomenon where organic and inorganic particulate impurities, scattering from the water, dissolved impurities and organic water cause intrinsic absorption which results in the loss of beam intensity. It should be noted that the amount of attenuation underwater shares a relationship with the Jerlov water type (Arst, 2003).
Absorption by Pure Seawater:
Seawater has a tendency to be absorbed primarily towards the red spectrum mainly because it contains Hydrogen Oxide molecules. With salts such as CaCl2, Na2SO4, NaCL, KCL and MgCl2 also present in the water, there is a considerably chance of light absorption taking place at specified wavelengths. Apart from the 400 to 500 nm window, as shown in Figure B, pure water is almost always absorptive (Shifrin, 1983). The amount of absorption occurring per meter of sea water is defined as the absorption coefficient of pure seawater. However, several other mechanisms such as the scattering from particulate and humic acids also play their part in the attenuation.
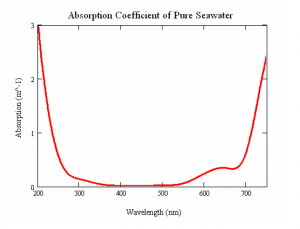
Figure 2: The absorption coefficient (m(-1)) is shown as a function of wavelength (nm) for pure seawater
CDOM Coloured Dissolved Organic Material and Suspended Particulate
Changes in the chemical concentration and compositions of the substances result in the variations in the spectral absorption of water. Depending on the properties of the water, these material substances are present in seawater in the form of suspended particulates (Shifrin, 1983).
Phytoplankton – Chlorophyll-a
Studies have revealed that Phytoplankton is the most significant factors influencing the amount of light received and reached to deep sea waters. The word Phytoplankton is derived from two words: planktos(wandering) and phyto (plant). Phytoplankton is found in deep waters (the euphotic zone) where only 1 percent of sun light manages to reach. As discussed before the typical depth of this zone is 100 meters but the exact figure is usually determined by taking several factors into account including the time of the day, location and weather. The presence of chlorophyll-a makes Phytoplakton grow in red and blue region and the mechanism eventually result in abundance of food production. The absorption of red and blue light increases with an increase in the concentration of this compound and causes the sea water to attain a light greenish colour (Kameda & Matsumura, 1988.
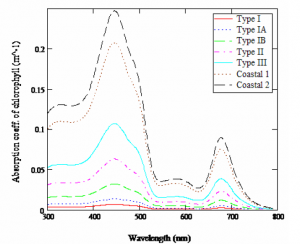
Figure 3: Cubic spline curve fitting of the absorption coefficient as a function of chlorophyll concentrations in the different Jerlov water types.
The distribution of phytoplankton in the euphotic zone is complex. Their distribution through the water column is not vertical despite the fact that several models are based around this assumption. The following given below can be used to determine the depth profile for chlorophyll.
(EQ-1)
Where,
B0 is the background chlorophyll concentration at the surface of the sea and is given in the units of mg/m3
C(z) is the concentration of chlorophyll a (mg/m3) at depth z (m)
h is the amount of chlorophyll above the background and is given in the units of mg/m2
S is the vertical gradient of chlorophyll concentration and is given in units of mg/m3/m
Zmax is the depth of the chlorophyll maximum (m)
Σ is the standard deviation of Gaussian distribution
The distribution changes dramatically with changes in the temperature and the concentration of nutrients as evident from Figure 4 below.
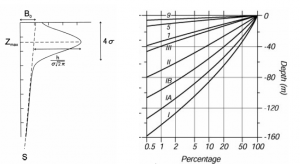
Figure 4: Chlorophyll depth profile, Attenuation of surface irradiance with Jerlov waters
It should be noted that the magnitude of the peak concentration amplifies closer to the shores where the position of the peak zmax moves closer to the top of the water column. The satellite remote data, as shown in the figure below can be used to calculate approximately the amount of chlorophyll in different types of water (Breves, 2000).

Figure 5: Satellite remote sensor reading of the cholorphyll – a concentration over the face of the earth [mg/m3]16
Table 1: Chlorophyll concentrations for different Jerlov Water types,
Concentration of Chlorophyll mg/m3 | Jerlov Water types |
12 | Coastal Water 2 |
9 | Coastal Water 1 |
3 | III |
1.25 | II |
.4 | IB |
0.1 | IA |
0.03 | I |
Colour Dissolved Organic Material
CDOM has the tendency of being converted into fulvic and humic acids and absorbed in the fluoresce and blue region at 420 to 450 nm mainly because it is made up of decaying organic marine matter. CDOM is also called the gelbstoff which means Yellow in German language. It has been observed that CDOM is present in very large quantities in the coastal waters whereas in the oceanic and deep blue concentration its concentration is on the lower side (Hansell, 2002).
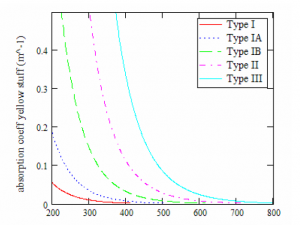
Figure 6: Absorption coefficient of gelbstoff, adapted from the one parameter model of gelbstoff as a function of chlorophyll
From the Figure 6 above, it is evident that the concentration of gelbstoff is directly proportional to the absorption coefficient. It is further proven that the concentration of gelbstoff is highly dependent on the location (Haltrin, 1998).
Scattering underwater:
The phenomenon where photons are redirected into new paths, thereby preventing the straight line on axis motion of photons is known as scattering under water. Scattering casts a shadow and therefore a beam will spread in loose light intensity or in diameter.
Scattering by Pure Seawater:
When estimating the overall scattering coefficient it is necessary to take into account the amount of initial scattering. This process should be followed at all times as water contains salts and scattering molecules. The Figure 7 below shows how wavelength and scattering coefficient of pure sea water are related to each other.
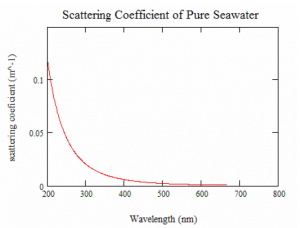
Figure 7: Scattering coefficient of pure seawater (m-1) as a function of wavelength (nm)
The equation given below is used to estimate the molecular scattering phase function when a photo experiences a change in direction due to a scattering event.
(EQ-2)
Where φ is the deviation in degrees from the original path
The βw(φ) function is the probability of a photon experiencing a path redirection by angle of φ due to photon scattering interaction with a water molecule (Haltrin, 1998). However, it should be noted that the above function is the combination of the back (angles between 90 and 180) scattering and forward scattering (angles between 0 and 90).
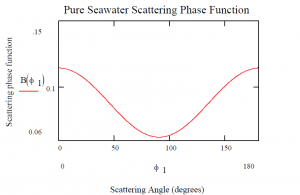
Figure 8: The angular distribution of the scattering phase function for pure water.
The forward scattering is likely to dominate the environments where scattering by particles in observed (Buchner, 1973). For example, the phase angle in clouds is estimated to be between 20 and 30 degrees and therefore the clouds are considered as a classic example of forward scattering. However, the phase angle is around only 7 degree in the oceanic environment.
Scattering may be present in the lower wavelengths in the ocean but it is absorption process that usually dominates the sea environment (Mclean et.al, 1998). Scattering can, however, play a significant role closer to the land where organic matter and particulate is brought into play by river runoff.
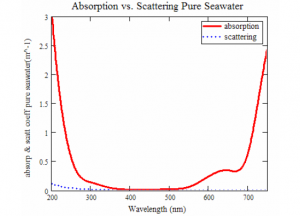
Figure 9: For pure seawater, the absorption coefficient is overlapped onto the scattering coefficient plot showing the dominate nature of absorption.
Scattering through Suspended Particulate:
As highlighted before, scattering can be brought about by both organic and inorganic particles flowing through the water column. Inorganic matter is the main cause of scattering in the coastal water just like scattering is caused by organic particles in the open ocean. It has proven that about 40 to 80 percent of the total scattering taking place in the coastal waters is caused by inorganic matter.
- Scattering by suspended particulates is dependent on the following factors:
- Reflection from the index of refraction
- The internal refraction
- The degree of external diffraction and reflection
The model used to represent the particulate scattering in this report is called the Kopelevich model and is given by the equation given below:
(EQ-3)
The possibility of forward scattering in the ocean water is considerably higher than the backscattering.
Turbulence:
The effects of temperature, salinity, variations from flow, variations of the refractive index, scintillation from turbulent water and the effects of stratified layers of waters are neglected for the purpose of this investigation. However, these factors can still have considerable second order effects but not as much as the primary factors which include scattering and absorption. Studies have confirmed that the water is incompressible. However, at certain pressure and depth, the refractive index does increase. The dependence of refractive index on environmental parameters have been measured by many researchers (Sanchez & McCormick, 2002).
The change in refractive index value in relation to the depth and pressure is negligible, with an increase of about 1.37 x 10 ^ 4 being recorded at 100 meters deep from the sea surface. Similarly, the temperature also shares a relationship with the refractive index with a decrease to 1.3318 measured at 30 degree centigrade. The effect of salinity is much greater with an increase of about 1.92 x 10 ^ - 4 estimated with one part per thousand increase in salinity. Although these effects can increase the challenge of tracking and pointing between under water platform, they are considered as secondary problems with main issues being the absorption, scattering and attenuation.
References:
Apel, J.R., (1987). Principles of Ocean Physic, pp509-584, International Geophysics Series, Vol.38, Academic Press
Arst, H., (2003). Optical Properties and Remote Sensing of Multicomponental Water Bodies, Praxis Publishing, Chichester, UK.
Breves, W. and Teuter, R., (2000). Bio-optical properties of gelbstoff in the Arabian Sea at
the onset of the southwest monsoon”, Earth Planet Science, 109, No. 4
Buchner, E.A., (1973). Computer Simulation of Light Pulse Propagation for Communication
Through Thick Clouds, Applied Optics, 12, 2391.
Lerner, (1973). Experiments on Light Pulse Communication and Propagation Through Atmospheric Clouds Appl. Opt.
Excerpted from: Ocean Optics Protocols for Satellite Ocean Color Sensor Validation,
Chapter 1 Sections 2 & 3, Rev 4, Volume IV
Garrison, T., (1996). Oceanography: An invitation to Marine Science, Chapter 10, Wadsworth
Publishing Company, Belmont
Hansell, D.A. and Carlson, C.A., (2002). Biogeochemistry of Marine Dissolved Organic Mater,
Academic Press, New York, pg 509-534.
Haltrin, V.I., (1999). Chlorophyll-based model of seawater optical properties, Appl. Opt.,38,No.33.
Jerlov,N.G., (1968). Optical Oceanography, Elsevier Publishing Company, New York, Vol. 5.
Kameda, T. and Matsumura, S., (1998). Chlorophyll Biomass off Sanriku, Northwestern Pacific,
Estimated by Ocean Color and Temperature Scanner (OCTS) and a Vertical Distribution
Model. National Research Institute of Far Seas Fisheries, Orido 5-7-1, Shimizu, Shizuoka
424-8633, Japan.
Mclean, J.W., Freeman, J.D and Walker, R.E., (1998). Beam Spread Function with Time Dispersion,Applied Optics, 37 4701.
Morel, A., (1984). Optical properties of pure water and pure sea water, in Optical Aspects of
Oceanography, edited by N. G. Jerlov and E. S. Nielsen, Academic Press, New York, USA.
Ocean Zonation, 2013. Available online at: http://www.aquatic.uoguelph.ca/oceans/Introduction/Zonation/zonation.htm [Retrieved on 02 December 2013]
Quan, X. and Fry, E.S., (1995). Empirical equation for the index of refraction of seawater," Appl. Opt.,34, 3477- 3480.
Sanchez, R. and McCormick, N.J., (2002). Analytic beam spread function for ocean
optics applications” Applied Optic.
Shifrin,K.S., (1983). Physical Optics of Ocean Water, American Institute of Physics, New York
Shifrin, K.S., (1988). Physical Optics of Ocean Water, American Institute of Physics, New York
Yentsch, C.S., (1960). The Influence of Phytoplankton Pigments on the Color of Sea Water,
Deep-Sea Res., 7, 1, (1960).
Get 3+ Free Dissertation Topics within 24 hours?